To be certified SANE, the following aspects will be assessed:
Brands can decide to certify one product, one line or their entire collection.

Certification processes are often unclear, expensive, and complicated. It can be discouraging for brands or production facilities to go through a certification process even for those already complying with all the requirements.
For that reason, we put great effort into designing a certification process that would be comprehensible, fast, and inexpensive as possible. We believe that brands and manufacturers should not need to call upon certification experts in order to get a product certified by SANE.
There are significant differences between SANE Standard and other certifications. Yet to minimize redundancy, SANE recognizes credible existing standards when their requirements are equivalent.
SANE will support applicants to prepare for the certification process.
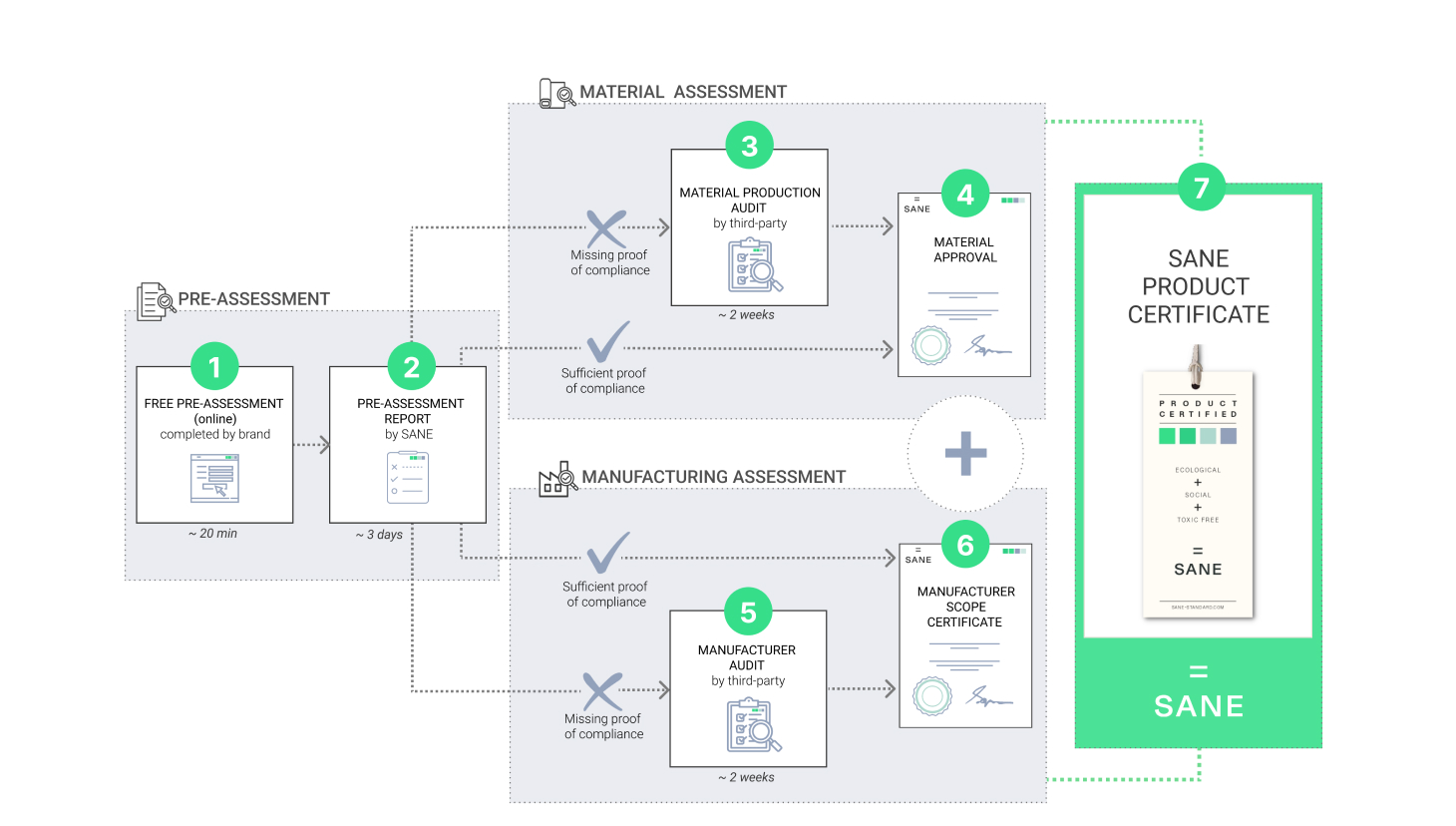
1.
The applicant fills in a free online questionnaire to determine if a product/unit is eligible for SANE certification. The applicant must send the completed product questionnaires to [email protected]
2.
SANE team members will assess if the company product(s) and/or manufacturing unit(s) are eligible to be certified. SANE may request additional documentation or proof of compliance during this stage. SANE then issues a report including eligibility, certification steps, estimated cost, and timeline. The report also specifies which manufacturing units need an online, partial, or complete audit and enclose a list of accredited third-party certifiers for the region.
3.
Unless the company can prove during the pre-assessment that the material fulfills SANE requirements through other accepted certifications, the material manufacturer(s) must be audited by a SANE-accredited third-party certifier.
The audit can be on-site, virtual, or based on documentation, depending on the proof of compliance provided during the pre-assessment.
If the outcome of the audit is successful, the material manufacturer will be granted a SANE Scope certificate.
SANE Scope Certificate is valid for one year. After 12 months, a control audit shall take place to reassess SANE requirements.
4.
If the company can provide sufficient proof of compliance (e.g.,: SANE Scope Certificate, or other accepted certification, etc.), the product is eligible to be certified SANE.
5.
Unless the company can prove during the pre-assessment that the end-product manufacturer fulfills SANE requirements through other accepted certifications, the manufacturer(s) must be audited by a SANE-accredited third-party certifier.
6.
If the outcome of the audit is successful, the manufacturer will be granted a SANE Scope certificate.
Alternatively, if sufficient proof of compliance is provided during the pre-assessment, a SANE Scope certificate will be issued without audit.
SANE Scope Certificate is valid for one year. After 12 months, a control audit shall take place to reassess SANE requirements.
7.
If sufficient proof showing that the product material and manufacturing comply with SANE requirements are provided, the product can be certified SANE.
The applicant will receive a SANE Product Certificate following the payment of the corresponding fee.
The Product Certificate is valid for one year. After one year, upon completion of volume reconciliation of the certified products production, the certificate can be extended if the production conditions remain unchanged.
For the products assessment and the payment of the certification fees, it is possible to combine products by Product Group if they are made of the same material, sourced from the same material supplier, and produced in the same facilities.
Upon request and a rapid assessment by SANE, products belonging to the same Product Group can be added to a Product Certificate after its release.